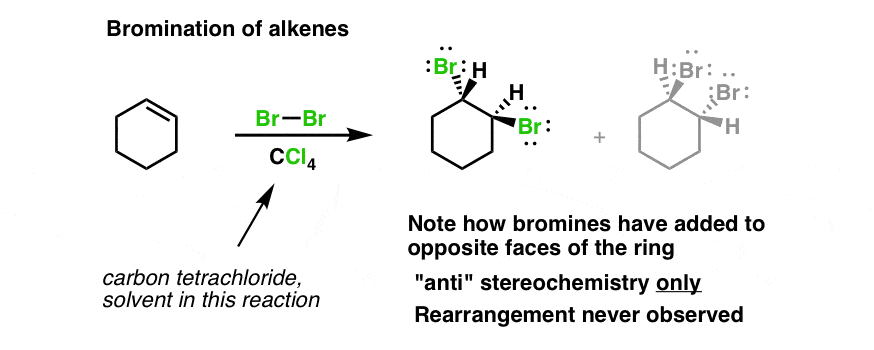
Reaction of Cyclohexene With Bromine
Colour of the bromine water solution is decolourized as it reacts with ethene. Bromine does not react with cyclohexane without UV light with or without water.
What Is The Equation Of The Reactions Between Cyclohexene With Br2 Ccl4 What Type Of Reaction Is This Quora
Consisting all single bonds.
. Many reactions of these aryl lithium and Grignard reagents will be discussed in later sections and the following equations provide typical. Esteves Synthesis 2010 2379-2382. The reaction is regioselective leading to Markovnikov-oriented products and the halofluorinated adducts follow anti-addition in the case of cyclohexene and 1-methylcyclohexene.
C 2 H 4. Check out a sample QA here. In the second step of the mechanism both H 2 O solvent and Br produced in the first step are nucleophiles and have the chance to react with the cyclic bromonium ion.
In recent years we have been investigating Cu-catalyzed methods for oxidation of styrenes and other alkenes for example 16 17 as well as CH bonds The former reactions are proposed to proceed via reaction of a benzylic radical intermediate with copperII generating an organocopperIII species that undergoes reductive elimination to afford a new CC or. C 4 H 8. This provides a powerful tool for the conversion of chloro bromo or iodo substituents into a variety of other groups.
Outline the mechanisms for these reactions. You are not reacting bromine with cyclohexane you are DISSOLVING bromine in cyclohexane and water mixed. 1-butene 2-butene and isobutylene.
A thought occurs to me. Want to see the full answer. The reaction of alkyl and aryl halides with reactive metals usually Li Mg to give nucleophilic reagents has been noted.
C 3 H 6. C 5 H 10. However since H 2 O is the solvent its concentration is much higher than that of Br so the major.
ZIF Zeolitic Imidazolate Framework is a metal organic framework MOF made by coordinated by four in the same way as Si and Al atoms are covalently joined by bridging oxygens in zeolitesThe sphere represents the pore size within the. Our modern society is based to a large degree on the chemicals we discuss in this chapter. The configuration of an unsaturated carbons include straight chain such as alkenes and alkynes as well as branched.
But liquid alkenes like cyclohexene react with bromine water solution in the. Journal of the American Chemical Society has been certified as a transformative journal by cOAlition S committing to a transition to 100 open access in the future. The use of bromine to test for unsaturation.
Draw one structure per sketcher. Reaction mass of 2-chloroethyl chloropropyl 2-chloroethylphosphonate mixture reaction mass of isomers and 2-chloroethyl chloropropyl 2-chloropropylphosphonate reaction mass of isomers 401-740-0 015-144-00-X reaction mass of pentyl methylphosphinate and 2-methylbutyl methylphosphinate 402-090-0 87025-52-3 015-145-00-5. Unsaturated hydrocarbons are hydrocarbons that have double or triple covalent bonds between adjacent carbon atomsThe term unsaturated means more hydrogen atoms may be added to the hydrocarbon to make it saturated ie.
The reaction takes place at room temperature if the reactants are in the gaseous state ethene. If your research funder has signed Plan S your open access charges may be. The ICSC project is a common undertaking between the World.
Figure 104e Formation of Halohydrin Figure 104f Mechanism. Students should be able to. This shows two layers with slightly different colours demonstrating that bromine is more soluble in non-polar solvents.
Interactive colour surface representations for the five d orbitals in 3D showing the nodes important for transition metal chemistry. Alkenes having four or more carbon atoms can form diverse structural isomersMost alkenes are also isomers of cycloalkanesAcyclic alkene structural isomers with only one double bond follow. Add additional sketchers using the drop- down menu in the bottom right corner.
The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. The primary aim of the cards is to promote the safe use of chemicals in the workplace. In Chapter 7 we noted that alkanessaturated hydrocarbonshave relatively few important chemical properties other than that they undergo combustion and react with halogensUnsaturated hydrocarbonshydrocarbons with double or.
Reaction of bromine water with cyclohexene. The formation of major and minor products in addition reactions of unsymmetrical alkenes. Consider the reaction of bromine with the pictured alkene.
Consider the reaction of bromine with the pictured alkene. Explain the formation of major and minor products by reference to the relative stabilities of primary secondary and tertiary carbocation intermediates. You do not have to consider stereochemistry.
The main target users are workers and those responsible for occupational safety and health. Most are made from petroleum. Students whove seen this question also like.
H H H H H wireframe labels Draw the organic products of reaction of the alkyne above with H3O in the presence of HgSO4.

Scheme 4 A Reaction Of Cyclohexene With Electrogenerated Bromine In Download Scientific Diagram
Reaction Of Bromine With Cyclohexane Cyclohexene And Benzene

Organic Chemistry Product Of Reaction Between Cyclohexene And Bromine In Methanol At 273 K Chemistry Stack Exchange
Comments
Post a Comment